Biomolecules with their important physical and chemical properties form a set with a lot of appeal for technological applications. Biomolecules and their mimetic structures can contribute to several technological processes due to their redox, catalytic, photochemical, and specific affinity properties. These properties can be used to generate electric current, as in fuel bio cells, capture solar energy to produce electricity and fuel, produce diagnostic biosensors, produce metallic nanoparticles, change material properties, among others. Here we present some works developed by our research groups that perfectly illustrate this diversity of applications.

Renal injury caused by renal ischemia and reperfusion strongly influences heart morphology, electrophysiology, and redox unbalance. The so-called cardiorenal syndrome is an important class of dysfunction since heart and kidneys are responsible for hemodynamic stability and organ perfusion through a complex network. In the present work we investigate the vibrational spectral features probed by Fourier-Transform Raman (FT-Raman) spectroscopy due to physiological alterations induced by renal ischemic reperfusion aiming to detect molecular markers related to progression of acute to chronic kidney injury and mortality predictors as well.
 C57BL/6J mice were subjected to unilateral occlusion of the renal pedicle for 60 min and reperfusion for 5, 8, and 15 days. Biopsies of heart and kidney tissues were analyzed.
C57BL/6J mice were subjected to unilateral occlusion of the renal pedicle for 60 min and reperfusion for 5, 8, and 15 days. Biopsies of heart and kidney tissues were analyzed.
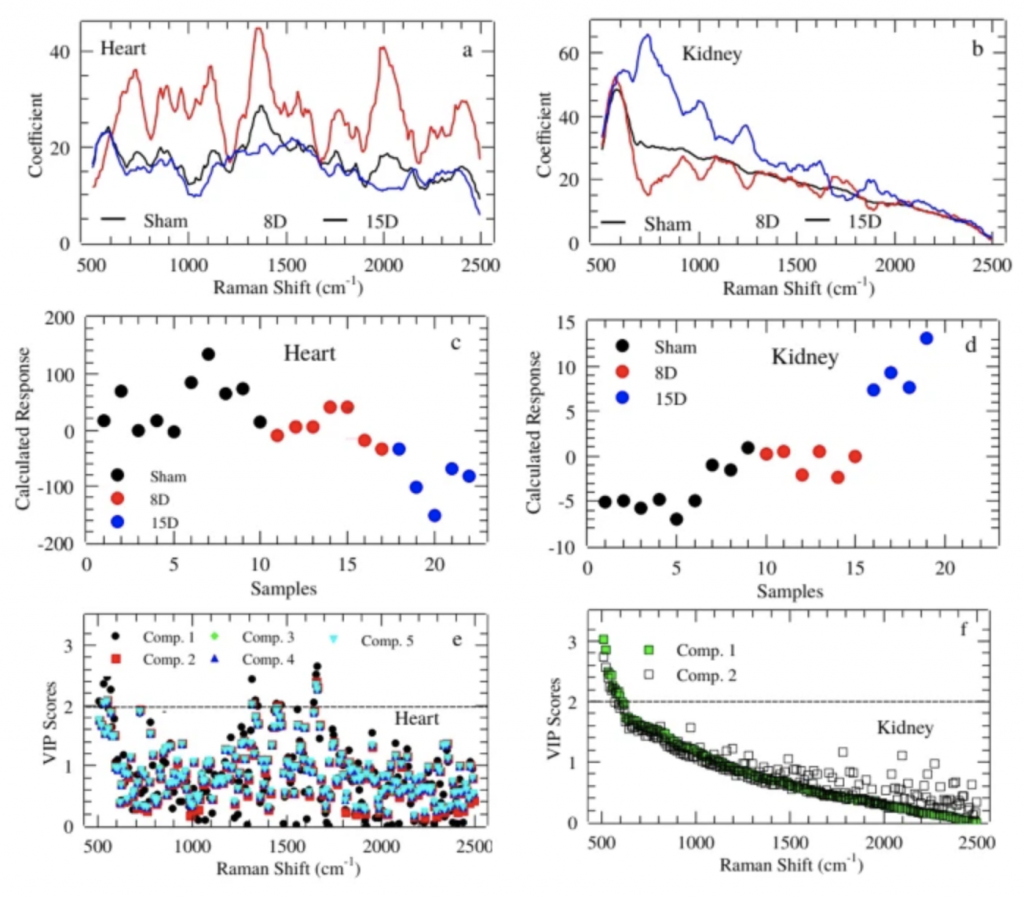
Our findings indicated that cysteine/cystine, fatty acids, methyl groups of Collagen, α-form of proteins, Tyrosine, and Tryptophan were modulated during renal ischemia and reperfusion process. These changes are consistent with fibroblast growth factors and Collagen III contents changes. Interestingly, Tyrosine and Tryptophan, precursor molecules for the formation of uremic toxins such as indoxyl sulfate and p-cresyl sulfate were also modulated. They are markers of kidney injury and their increase is strongly correlated to cardiovascular mortality.
Regarding this aspect, we notice that monitoring the Tyrosine and Tryptophan bands at 1558, 1616, and 1625 cm−1 is a viable and and advantageous way to predict fatality in cardiovascular diseases both “in vivo” or “in vitro”, using the real-time, multiplexing, and minimally invasive advantages of FT-Raman spectroscopy.
https://www.nature.com/articles/s41598-021-93762-z
The figure represent the regression coefficient in PLS-DA for Heart (a) and Kidney (b). Calculated response for heart (c) and kidney (d) using up to 5th and 2th component, respectively.Average PLS-DA Variable Importance in Projection (VIP) for those components with best classification performance for Heart (e) and Kidney (f).

Oral squamous cell carcinoma (OSCC) represents 90% of oral malignant neoplasms. The search for specific biomarkers for OSCC is a very active field of research contributing to establishing early diagnostic methods and unraveling underlying pathogenic mechanisms.
In this work we investigated the salivary metabolites and the metabolic pathways of OSCC aiming find possible biomarkers. Salivary metabolites samples from 27 OSCC patients and 41 control individuals were compared through a gas chromatography coupled to a mass spectrometer (GC-MS) technique.

Figura. Heatmap usando dados de PCA para OSCC e classes de controle.
Our results allowed identification of pathways of the malate-aspartate shuttle, the beta-alanine metabolism, and the Warburg effect. The possible salivary biomarkers were identified using the area under receiver-operating curve (AUC) criterion. Twenty-four metabolites were identified with AUC > 0.8. Using the threshold of AUC = 0.9 we find malic acid, maltose, protocatechuic acid, lactose, 2-ketoadipic, and catechol metabolites expressed.
We notice that this is the first report of salivary metabolome in South American oral cancer patients, to the best of our knowledge.
Our findings regarding these metabolic changes are important in discovering salivary biomarkers of OSCC patients. However, additional work needs to be performed considering larger populations to validate our results.
https://www.mdpi.com/2218-1989/11/10/650/htm


The search for cleaner and cheaper production processes with safer and more efficient synthetic solutions is part of the challenges faced by the pharmaceutical industry.
To overcome these challenges, mechanochemistry has been explored as a way to provide not only sustainable processes employing solvent-free synthesis (or minimal amounts of solvents) but also to obtain materials that are sometimes difficult or even impossible to produce from conventional solution-phase routes. Furthermore, mechanochemistry has emerged as a powerful tool to rapidly and efficiently search for different polymorphic forms and cocrystals as well as for the discovery and development of new drugs.
Mechanochemistry refers to a chemical reaction induced by mechanical energy involving solids. This method provides several advantages over solution-phase syntheses, such as minimizing the need for large volumes of solvents in chemical reactions and greener and more efficient synthetic solutions. In this paper we obtain, via mechanochemistry, a hybrid salt, named MEFAS, derived from two antimalarial molecules – mefloquine and artesunate.
We demonstrate, using a simple experimental procedure, how the catalytic amount of liquid present during mechanochemical reactions is decisive to obtain MEFAS. Fourier-transform infrared spectroscopy (FTIR) and nuclear magnetic resonance (NMR) data indicate that liquid-assisted mechanochemical reactions are promising in the formation of the hybrid salt, which is formed via a hydrogen interaction of the carboxylate group of the artesunate molecule with the piperidine group of mefloquine.
https://pubs.rsc.org/en/content/articlehtml/2019/gc/c9gc02478f
Bioactive glasses containing rare earth elements have been proposed as promising candidates for applications in brachytherapy of bone cancer. However, their safety relies on a proper dissolution to avoid radioactive materials in the human body, and desirable bioactive properties to regenerate the bone defect caused by the tumor.

Brachytherapy is a type of radiotherapy treatment that consists of delivering doses of radiation directly to the tumor site. Such proximity between the radiation source and the tumor site increases the effectiveness of the treatment, besides avoiding healthy tissues to be damaged by unneeded radiation doses when compared to external beam radiotherapy procedures [1]. The biological effect of β-ray radiation includes DNA and other biomolecules damages, leading to cell apoptosis. Besides, when brachytherapy is combined with other therapies, a synergetic effect can be observed, contributing to a faster patient recovery [2,3].
In this work, we proposed a new series of sol-gel-derived bioactive glasses containing holmium oxide, based on the system (100-x)(58SiO2-33CaO-9P2O5)-xHo2O3 (x = 1.25, 2.5 and 5 wt%). The glasses were characterized regarding their dissolution behavior, bioactivity, and cytotoxicity with pre-osteoblastic cells. Also, in the dissolution experiments, the Arrhenius and Eyring equations were used to obtain some thermodynamic properties of glass dissolution.
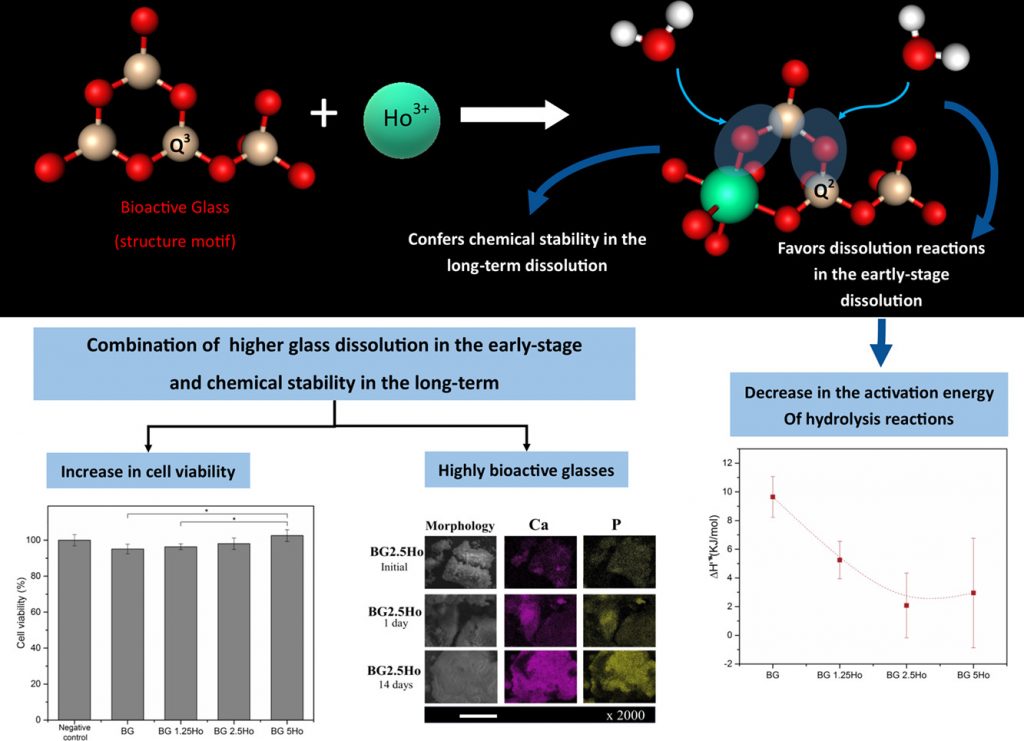
The results evidenced that the addition of holmium ions in the glass structure decreased the energy barrier of hydrolysis reactions, which favors glass dissolution in an early-stage. However, in the long-term, the strength of Si-O-Ho bonds may be the cause of more stable dissolution. Besides, glasses containing holmium were as bioactive as the 58S bioactive glasses, a highly bioactive composition. Cytotoxicity results showed that all glasses were not cytotoxic, and the composition containing 5 wt.% of Ho2O3 enhanced cell viability.
Finally, these results suggest that these glasses are suitable materials for brachytherapy applications due to their proper dissolution behavior, high bioactivity, and high cell viability.
https://www.sciencedirect.com/science/article/pii/S092849312033513X
Nanocellulose-based foams are widely used as absorbents due to their highly porous networks and large surface areas. However, their inherent hydrophilicity and poor wet resilience limit their applications in water remediation for the removal of hydrophobic components (e.g., oil and organic solvent spills). A straightforward process combining biomass-derived materials, cellulose nanofibrils (CNFs), and natural rubber (NR) latex, in the absence of any organic solvent, is herein elucidated to design eco-friendly hydrophobic foams.

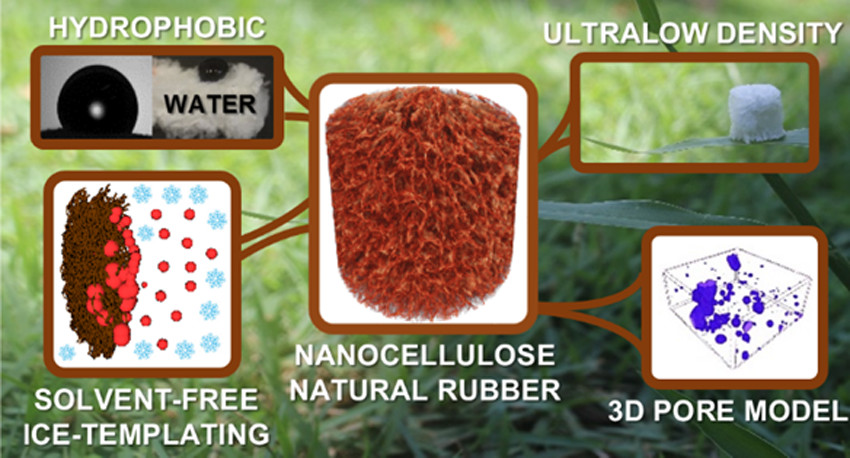
Aqueous NR and CNF dispersion exhibited great colloidal stability (ζ-potential above −30 mV), a desirable characteristic to obtain controlled structures. The spherical shape of NR (diameter of ca. 500 nm) and the fibrous morphology of CNF, both by themselves and when mixed, were evidenced by cryogenic transmission electron microscopy.
The porous architecture built up through ice templating was corroborated by scanning electron microscopy and 3D X-ray microtomography (μCT). The CNF 3D network and NR promoted rigid and robust foams with elevated compressive modulus (∼600 kPa, for NR20/CNF80). In situ four-dimensional (4D) μCT revealed an impressive increase in oil uptake—from 44 ± 5 to 90 ± 7%—by foam pores upon NR addition.
NR was demonstrated by pore segmentation to be evenly distributed throughout the CNF surface, awarding great hydrophobicity (apparent contact angle of around 100°), excellent and quick oil/organic solvent absorption (above 50 g·g–1 in 3 s), and excellent reusability (20 cycles) for organic solvents and oils.
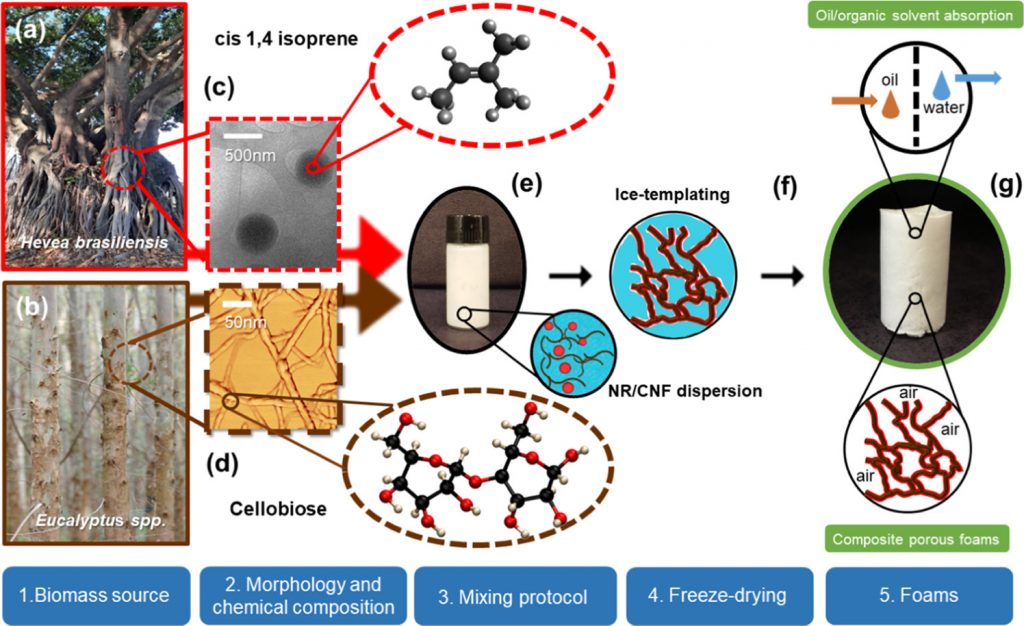
Schematic procedure to obtain natural rubber (NR) latex and cellulose nanofibrils (CNF) foams. Digital photographs of (a) Hevea brasiliensis and (b) Eucalyptus spp. biomass source of NR and CNF, respectively. (c) Cryogenic transmission electron microscopy (cryo-TEM) image of a colloidal dispersion of extracted NR particles and the chemical structure of the NR monomer (cis-isoprene). (d) Atomic force microscopy (AFM) image of CNF dispersion and the chemical structure of the main unit of cellulose (β-d-glucopyranose or cellobiose).(30) (e) NR and CNF dispersion: zoomed circles show illustrations of the NR (red) and CNF (brown) dispersion. (f) Illustration of the porous structure being formed by the ice-templating process. (g) Digital photograph of NR/CNF foams.
This green material exhibits great capacity of soaking nonpolar molecules, standing out as an environmentally friendly, cost-effective, and promising alternative for water cleanup.
https://pubs.acs.org/doi/10.1021/acsanm.0c02203?fig=fig1&ref=pdf&#
Pickering emulsions are emulsions stabilized by solid particles rather than surfactants. The particle stabilization offers specific properties to these colloidal systems, such as high resistance to coalescence at low solid content due to the strong particle adsorption onto the interface between oil and water. Besides, this “surfactant-free” character is especially attractive for applications in cosmetic and pharmaceutical products, where surfactants can cause adverse effects (irritancy, hemolytic behavior, etc.).
The Pickering emulsions can meet the current demand for ecofriendly products using particles derived from renewable and sustainable sources, such as nanocelluloses.
Our results demonstrated a facile and efficient protocol to prepare stable O/W Pickering emulsions, using renewable nanomaterials without other co-stabilizers, such as salts, polymers and/or surfactants. These results can be extended to other oil phases, as hydrocarbons, and probably to any negatively dispersed phase.
Hydrophobic oleic acid/water interfaces are negatively charged. Hence, the use of cationic nanocelluloses as stabilizers of Pickering emulsions could improve the colloidal stability due to the electrostatic complexation at the oil-water interface.
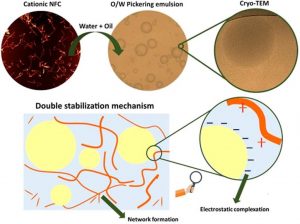
In this study, two cationic nanofibrillated cellulose (cNFCs) with two degrees of substitution were prepared and used as stabilizers of Pickering emulsions.
The adsorption of cNFCs at the oil: water interface was evaluated by interfacial tension, atomic force microscopy, and centrifugation measurements. LUMiSizer and optical microscopy techniques were used to analyze the colloidal stability and oil droplets morphology, respectively. Besides, the rheological behavior of the continuous aqueous phase was determined through flow and stress sweep curves. Finally, the dispersion of cNFCs in a diluted emulsion was visualized by cryogenic transmission electron microscopy (cryo-TEM).
Cationic NFCs were more efficient in partitioning to the oil:water interface compared to their anionic analogous, oCNF. The electrostatic attraction between the positively charged trimethylammonium groups and the negatively charged deprotonated oleic acid reduced the interfacial tension and improved the colloidal stability of O/W Pickering emulsions. cNFCs dispersed in the aqueous phase were found to increase the viscosity, decelerating the oil drops coalescence. Therefore, the stabilization of cNFCs Pickering emulsions had a synergistic effect from the electrostatic complexation at the liquid-liquid interface and network formation in the aqueous phase, as visualized by cryo-TEM.
Despite the growing interest of the automotive industry in using recycled polymers, their undesired odor is limiting their application in vehicles’ interior components. To get deeper insights into its causes, this study aimed at characterizing the odor of post-consumer and recycled automotive polypropylene with different contents of talc and an anti-fogging additive.

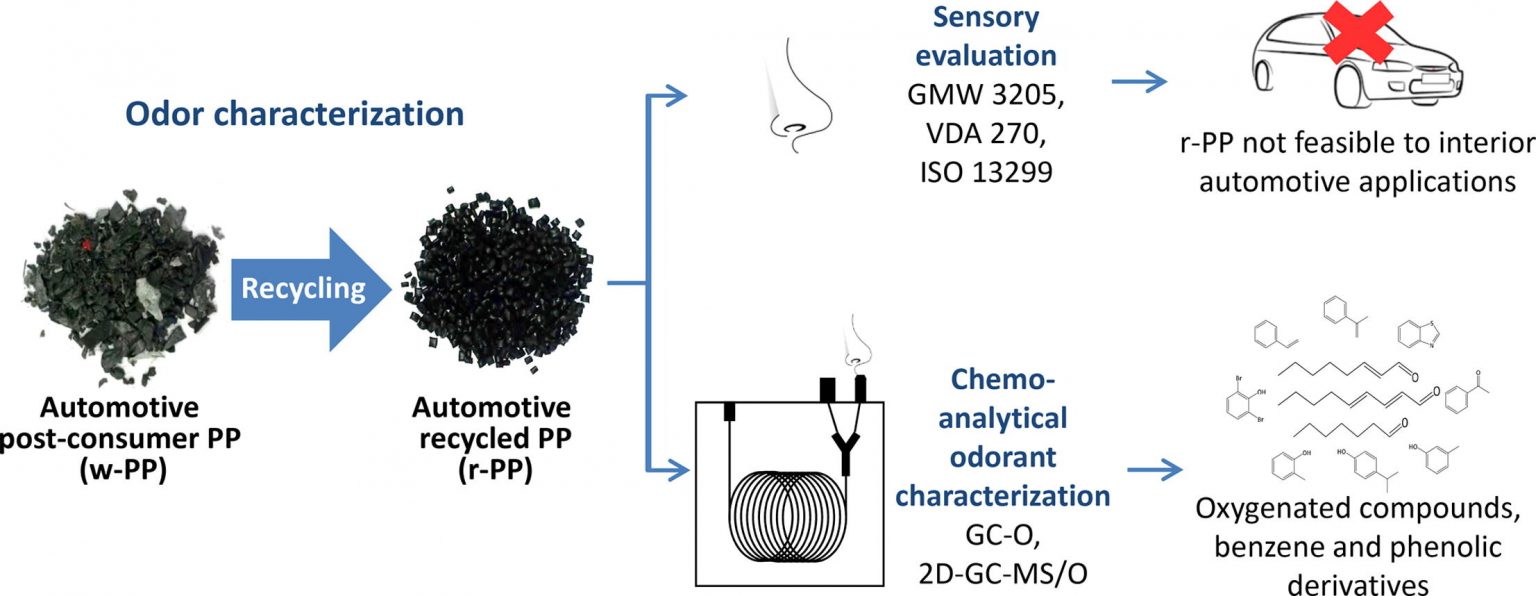
Samples were evaluated by different sensory methods currently applied by the automotive industry (GMW 3205 and VDA 270), which confirmed, that they are not feasible for reuse in interior automotive applications. As these odor evaluations are usually performed by non-trained panelists and do not allow a detailed description of the samples’ single odor qualities, sensory evaluation according to ISO 13299 was performed by trained panelists.
Samples showed medium–high odor intensities rated from 5.1 to 5.6, and a general dislike of the odor with hedonic ratings from 1.8 to 2.6 (scale 0–10). Their odor profiles correlated well with the odorants identified by chemo-analytical characterization using gas chromatography–olfactometry (GC-O) and two-dimensional GC-O coupled with mass spectrometry (2D-GC–MS/O).
An array of odorants with benzene and phenolic structures were identified as potential contributors to the samples’ overall smell and are likely to originate from degradation of additives commonly used in automotive components.
While the addition of talc or anti-fogging additive did not significantly improve the odor of the samples, the description of the samples’ smell and the identification of odor-active compounds related to it allow the development of avoidance strategies for the manufacturing of neutral smelling products intended for vehicles’ interior applications.
https://www.sciencedirect.com/science/article/pii/S0956053X20303871
Phenothiazines are a class of molecules that have been applied in antipsychotic therapy, photodynamic therapy, dyes, sunscreen, and more recently light-harvesting for energy applications. In the present study, the photochemical properties of phenothiazine aggregates were used for oxidative catalysis and synthesis of gold nanoparticles with potential applications in Boolean logic operations.

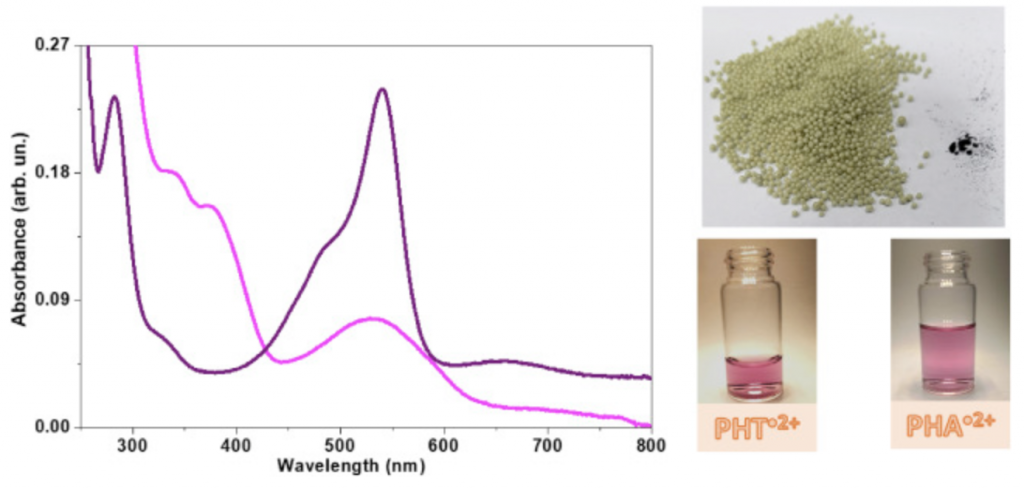
Phenothiazines have the peculiar photochemical property, which is the stabilization of the photochemically-generated radical cation in their supramolecular aggregates. The phenothiazine radical cations exhibit pink color related to a broad visible spectral band with a peak at 520 nm.
In the present study, the pair radical cation/reduced form of two phenothiazines, 10H-phenothiazine (PHT) and 3,7-bis (phenylamino) phenothiazine-5-iodide, named here phenoaniline (PHA) were photochemically generated and characterized by absorbance with a peak at the range of 520–550 nm, direct and spin-trapping EPR measurements. 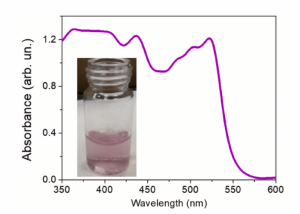
In the present study, the pair radical cation/reduced form of two phenothiazines, 10H-phenothiazine (PHT) and 3,7-bis (phenylamino) phenothiazine-5-iodide, named here phenoaniline (PHA) were photochemically generated and characterized by absorbance with a peak at the range of 520–550 nm, direct and spin-trapping EPR measurements.
The radical cation was separated from the reduced form using the resin styrene-divinylbenzene copolymer containing iminodiacetate ions (Chelex 100®). The radical cation entrapped in the resin remained stable and could be used for catalytic oxidation processes. The reduced form remained in aqueous solution and had its reducing properties used to produce gold nanoparticles.
Reduced PHT produced gold nanoparticles with dimensions around 7 nm that were arranged in monodispersed supramolecular aggregates of ~60 nm, stabilized by oligomerized phenothiazine, whereas PHA formed anisotropic gold nanoparticles.
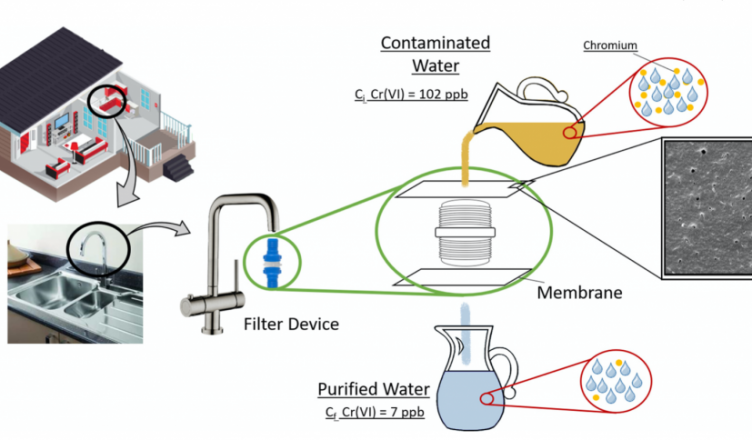
The removal of chromium from contaminated water was investigated using biodegradable membranes and functionalized cellulose nanoparticles.

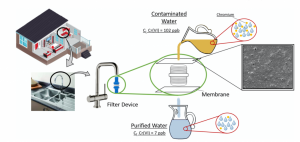 A small device for domestic use was tested to confirm the efficiency of the membranes, and the functionalized membranes showed 71% of chromium removal, which highlights its potential for water decontamination.
A small device for domestic use was tested to confirm the efficiency of the membranes, and the functionalized membranes showed 71% of chromium removal, which highlights its potential for water decontamination.
- Cellulose nanostructures (CNS) were phosphorylated and improved the Cu adsorption.
- Biodegradable membranes of PBAT were prepared via inversion phase technique.
- The CNS improved the mechanical properties of PBAT membranes.
- Membranes with phosphorylated CNS showed better chrome removal from water samples.
We used poly(butylene adipate-co-terephthalate), a flexible polymer, for membrane development via phase inversion technique. CNS, with and without phosphorylation (CNS-P), was used aiming to improve the removal of chromium from contaminated drinking water. The neat CNS showed limited efficiency on the Cr removal. On the other hand, CNS-P indicated removal of 93% and 88% of Cr(VI) and Cr(III), respectively. The Cr removal can be associated with CNS higher surface area and active sites, which allows more regions for immobilization of chromium species. Also, at lower pH the CNS-P presents a surface charge that can interact via electrostatic forces with the chromium species, which increases the removal efficiency. CNS-P also showed greater thermal stability due to phosphorylation. The addition of CNS and CNS-P improved the membranes’ mechanical performance, which improves the applicability of this material, with potential application in domestic houses and water treatment stations.
Solid-state ionic conductor is an essential and critical part of electrochemical devices such as batteries and sensors. Nano-sized silver iodide (AgI) is the most promising ionic conductor due to its superionic conductivity at room temperature. In recent years, proteins have been used as organic templates to obtain high-performance solid-state ionic conductors as well as to extend their applications in a biosensor. Here, we report the unprecedented ultrafast synthesis of thermally stable protein-coated AgI nanoparticles (NPs) through the photo-irradiation method for solid-state electrolyte.

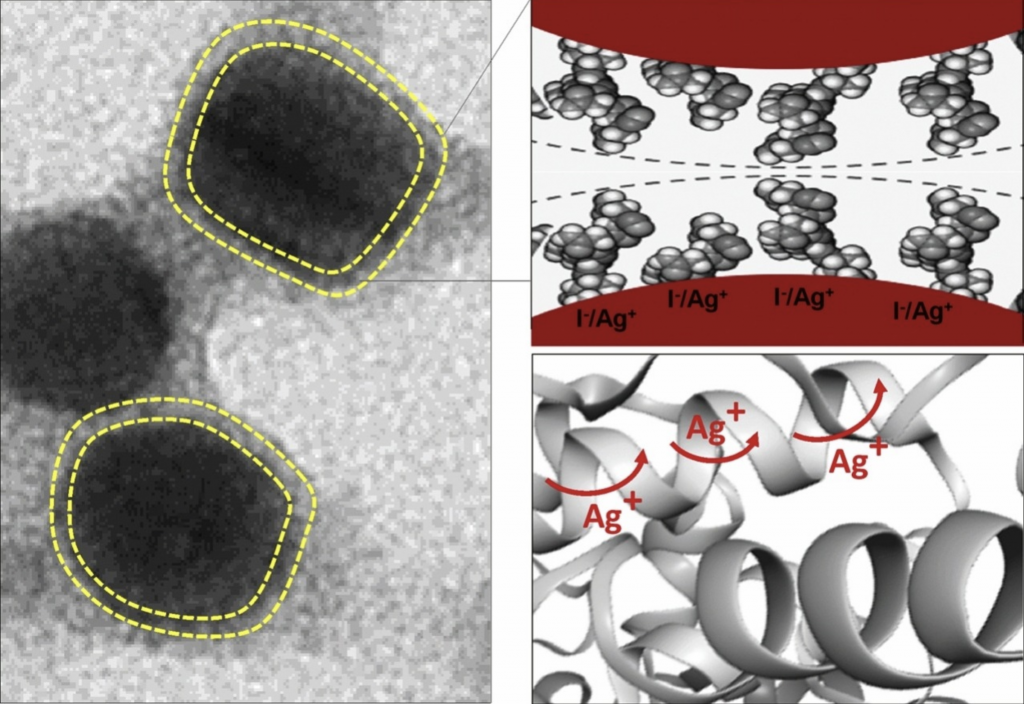 The synthesis was performed using a hyperthermostable bacterial β-glucosidase. The protein-coated AgI NPs with an approximate diameter of 13 nm showed that the controllable transition from the α- to β-/γ-phase was drastically suppressed down to 41 °C in the cooling process. After drying, the product represents a thermally stable organic-inorganic hybrid system with superionic conductivity. It is noteworthy that the superionic conductivity (σ ˜ 0.14 S/cm at 170 °C) of thermally stable protein-coated AgI NPs is maintained during several thermal cycles (25–170 °C). To our knowledge, this is the first report showing the diffusion of mobile Ag+ ions on the surface of the AgI NPs through a protein matrix. The facile synthesis method and high performance of the protein-coated AgI NPs may provide a latent application in the mass production of nanobatteries and other technological applications.
The synthesis was performed using a hyperthermostable bacterial β-glucosidase. The protein-coated AgI NPs with an approximate diameter of 13 nm showed that the controllable transition from the α- to β-/γ-phase was drastically suppressed down to 41 °C in the cooling process. After drying, the product represents a thermally stable organic-inorganic hybrid system with superionic conductivity. It is noteworthy that the superionic conductivity (σ ˜ 0.14 S/cm at 170 °C) of thermally stable protein-coated AgI NPs is maintained during several thermal cycles (25–170 °C). To our knowledge, this is the first report showing the diffusion of mobile Ag+ ions on the surface of the AgI NPs through a protein matrix. The facile synthesis method and high performance of the protein-coated AgI NPs may provide a latent application in the mass production of nanobatteries and other technological applications.
See more in https://www.sciencedirect.com/science/article/abs/pii/S0927776518309391
 Pineapple crown is an important source of cellulose that is still going to waste because of the lack of knowledge about their economic uses. The isolation of cellulose nanocrystals (CNC) from pineapple crown leaf (PCL) wastes arises as an important alternative to use PCL wastes in high value-added applications, and has not been reported yet.
Pineapple crown is an important source of cellulose that is still going to waste because of the lack of knowledge about their economic uses. The isolation of cellulose nanocrystals (CNC) from pineapple crown leaf (PCL) wastes arises as an important alternative to use PCL wastes in high value-added applications, and has not been reported yet.
In this study, CNC were successfully extracted from PCL wastes using chemical treatments followed by acid hydrolysis using sulfuric acid.

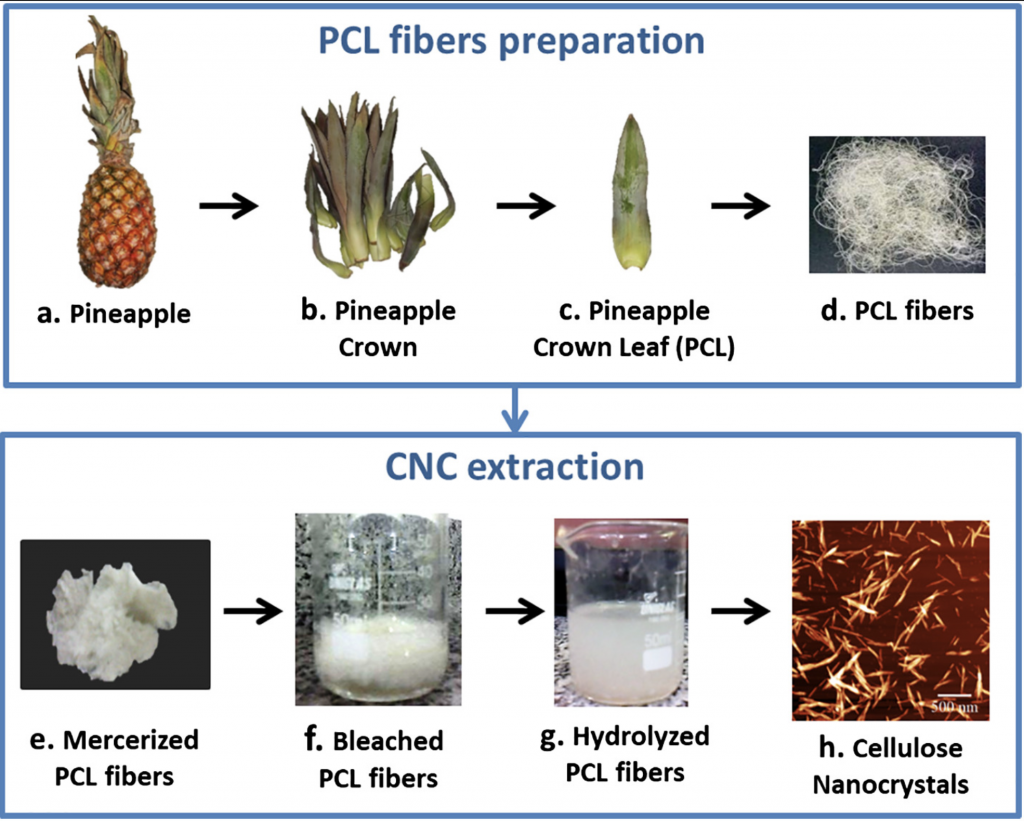 FTIR results confirmed the removal of the non-cellulosic compounds of PCL through the mercerization and bleaching treatments.
FTIR results confirmed the removal of the non-cellulosic compounds of PCL through the mercerization and bleaching treatments.
SEM and AFM showed that the diameter of PCL fibers was reduced from 18 μm to 39 nm after the hydrolysis reaction, resulting in CNC with rod-like shape.
The obtained CNC showed cellulose I crystalline structure with high crystallinity index (73%).
The thermal degradation of CNC started at 124 °C, what was attributed to the presence of surface sulfate groups identified by elemental analysis.
The high hydrophilicity of CNC was verified by its high moisture content and absorption.
The results showed that the CNC isolated from PCL have interesting properties to be used in many liquid media applications, besides their use as reinforcement in nanocomposites.
See more in https://www.sciencedirect.com/science/article/pii/S014181301833976X
Nowadays, cellulose nanostructures (CNS) have attracted considerable attention on the development of green bio-based and biodegradable materials. It has been occurred because this biopolymer is the most abundant and available biomaterial on the planet. Cellulose can be found in the form of nanoscale microfibrils in the plant cell wall (Cunha, Zhou, Larsson, & Berglund, 2014). Recently, the use of cellulose has been widely studied due to its sustainability, renewability characteristics, besides the low-cost and high-abundance. Furthermore, nanotechnology has attracted attention for development of advanced materials with properties such as high surface area, low density (i.e. low weight material for the volume occupied – generates lower transport costs and is lighter than non-polymeric materials) and high mechanical strength (Du et al., 2016).
In this study a homogeneous system for nanocellulose acetylation was investigated.

 Cellulose nanostructures (CNS) were obtained from microcrystalline cellulose, and modification was conducted to increase hydrophobicity and physico-chemical properties of the nanoparticles. Sulfuric acid and hydrochloric acid were used for the isolation. The CNS were characterized with dynamic light scattering, zeta potential, atomic force microscopy, Fourier-transform infrared spectroscopy, nuclear magnetic resonance, X-ray photoelectron spectrometry, degree of substitution (DS), X-ray diffraction (XRD), and thermogravimetric analysis. Nanostructures obtained with sulfuric acid showed lower particle sizes and lower thermal stability. After modification, the results indicated the substitution of OH groups in cellulose structure by acetyl groups. The XRD patter was considerably modified and it was verified that acetylation increased the thermal stability. Different methods were used to calculate the DS, and the differences between the methodologies were explained. The acetylated samples play an important role in the nanocomposites field, since the hydrophobic surface increase its applications.
Cellulose nanostructures (CNS) were obtained from microcrystalline cellulose, and modification was conducted to increase hydrophobicity and physico-chemical properties of the nanoparticles. Sulfuric acid and hydrochloric acid were used for the isolation. The CNS were characterized with dynamic light scattering, zeta potential, atomic force microscopy, Fourier-transform infrared spectroscopy, nuclear magnetic resonance, X-ray photoelectron spectrometry, degree of substitution (DS), X-ray diffraction (XRD), and thermogravimetric analysis. Nanostructures obtained with sulfuric acid showed lower particle sizes and lower thermal stability. After modification, the results indicated the substitution of OH groups in cellulose structure by acetyl groups. The XRD patter was considerably modified and it was verified that acetylation increased the thermal stability. Different methods were used to calculate the DS, and the differences between the methodologies were explained. The acetylated samples play an important role in the nanocomposites field, since the hydrophobic surface increase its applications.
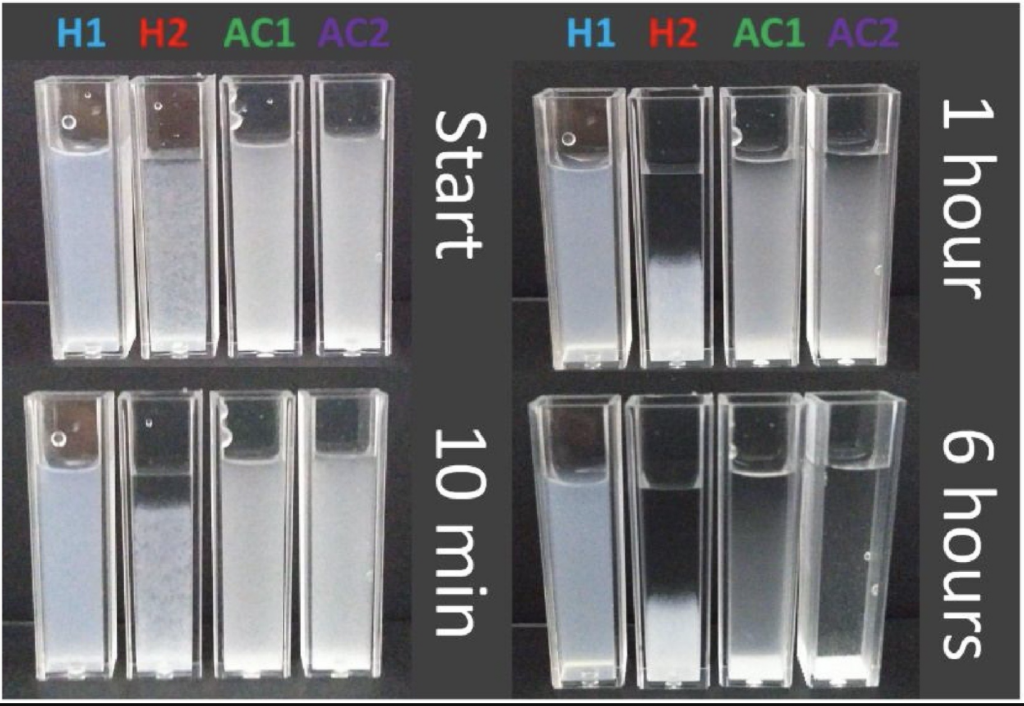
The figure on the left shows the stability behavior of the suspensions, with time variation. For the H1 sample it was not possible to observe a significant change in the system, even after 6 h of follow-up, which is a high indication of the stability of the suspension. On the other hand, the H2 sample presents visual alteration with 10 min where the sample begins to agglomerate, demonstrating the low stability in aqueous suspension. This result corroborates the values of the zeta potential, where a greater potential (in modulus) was observed for the H1 sample and can be associated with the presence of sulfate groups and hydroxyl groups, for H1 and H2 respectively. The samples AC1 and AC2, despite the presence of acetyl groups, were still well dispersed in aqueous suspension for periods of up to 1 h, and above that period it is observed that the system begins to agglomerate and decant. This result corroborates the indicative that although it does not present electrostatic stability, the system presents a mechanism of steric stability by the presence of the acetyl groups, since the suspension was shown stable for low times.
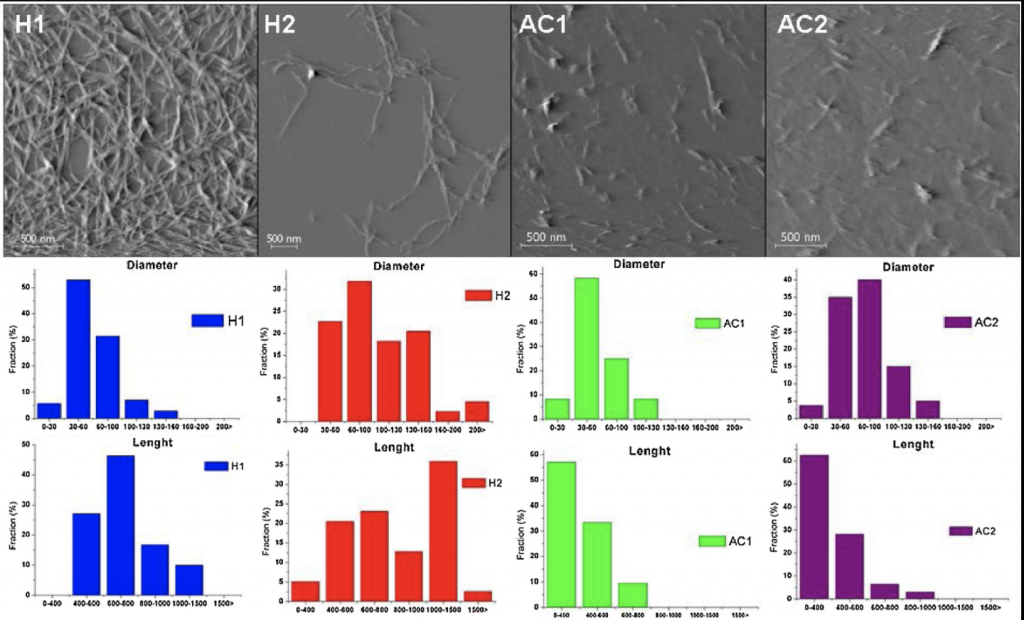 On the right, the analysis shows AFM images of the obtained nanoparticles, which was used to evaluate in greater detail the structures formed. The action of both acids allowed the isolation of CNS with a very homogeneous morphology, and they have an elongated typical shape of cellulose nanofibers. Usually for the acid hydrolysis is observed the formation of cellulose nanocrystals, but it is also reported that under non-drastic reaction conditions it is possible to obtain nanofibers, as was the case (Corrêa, de Teixeira, Pessan, & Mattoso, 2010). It was observed that the samples H1 and H2 showed a L/D of 11.5 and 8.6 for H1 and H2, respectively. These values and the shape of nanofibers is consistent with the DLS result which presented values with great variation.
On the right, the analysis shows AFM images of the obtained nanoparticles, which was used to evaluate in greater detail the structures formed. The action of both acids allowed the isolation of CNS with a very homogeneous morphology, and they have an elongated typical shape of cellulose nanofibers. Usually for the acid hydrolysis is observed the formation of cellulose nanocrystals, but it is also reported that under non-drastic reaction conditions it is possible to obtain nanofibers, as was the case (Corrêa, de Teixeira, Pessan, & Mattoso, 2010). It was observed that the samples H1 and H2 showed a L/D of 11.5 and 8.6 for H1 and H2, respectively. These values and the shape of nanofibers is consistent with the DLS result which presented values with great variation.
See more in https://www.sciencedirect.com/science/article/abs/pii/S0144861719304722?via%3Dihub
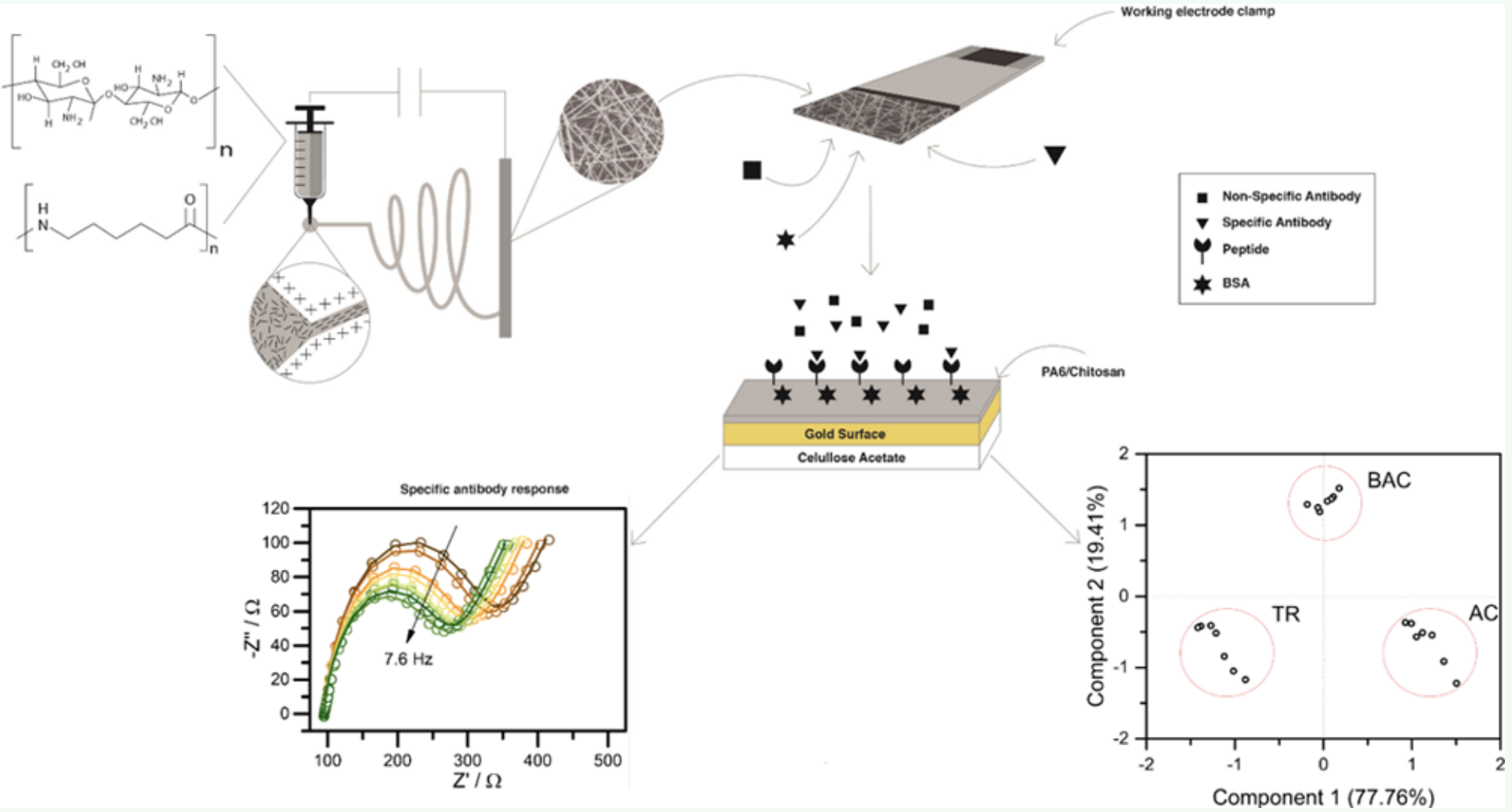
Prof. Wendel Alves’s research group, in partnership with Rheabiotech Ltda, has developed a biosensor that uses a chitosan-based polyamide-6 polymer blend to immobilize an L. infantum-specific antibody recognition peptide sequence (ACS Appl. Electron. Mater. 2019, DOI: 101021/acsaelm.9b00476).
https://pubs.acs.org/doi/10.1021/acsaelm.9b00476
The blend was chosen due to the excellent properties of chitosan (biocompatibility) and polyamide (mechanical properties), being used in the immobilization of biomolecules, which are incorporated by electrostatic entrapment, preserving their structure and therefore their bioactivity even when compared to materials that are already used for such function. The work was promising, where it was possible to detect concentrations of the order of 1 pg mL-1 (while the ELISA reached 1 ng mL-1), discriminating clearly and objectively human serum samples contaminated with visceral leishmaniasis and healthy humans.
Vibrational spectroscopy has been widely employed to unravel the physical-chemical properties of biological systems. Due to its high sensitivity to monitoring real time “in situ” changes, Raman spectroscopy has been successfully employed, e.g., in biomedicine, metabolomics, and biomedical engineering. The interpretation of Raman spectra in these cases is based on the isolated macromolecules constituent vibrational assignment. Due to this, probing the anharmonic or the mutual interactions among specific moieties/side chains is a challenge.
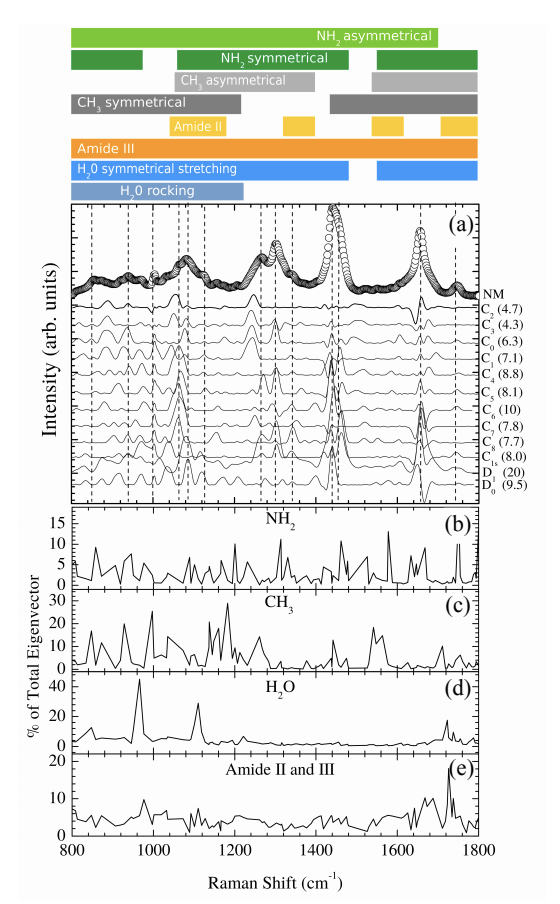 We present a complete vibrational modes calculation for connective tissue in the fingerprint region (800 – 1800 cm−1) using first-principles density functional theory. Our calculations accounted for the inherent complexity of the spectral features of this region and useful spectral markers for biological processes were unambiguously identified. Our results indicated that important spectral features correlated to molecular characteristics have been ignored in the current tissue spectral bands assignments. In particular, we found that the presence of confined water is mainly responsible for the observed spectral complexity.
We present a complete vibrational modes calculation for connective tissue in the fingerprint region (800 – 1800 cm−1) using first-principles density functional theory. Our calculations accounted for the inherent complexity of the spectral features of this region and useful spectral markers for biological processes were unambiguously identified. Our results indicated that important spectral features correlated to molecular characteristics have been ignored in the current tissue spectral bands assignments. In particular, we found that the presence of confined water is mainly responsible for the observed spectral complexity.
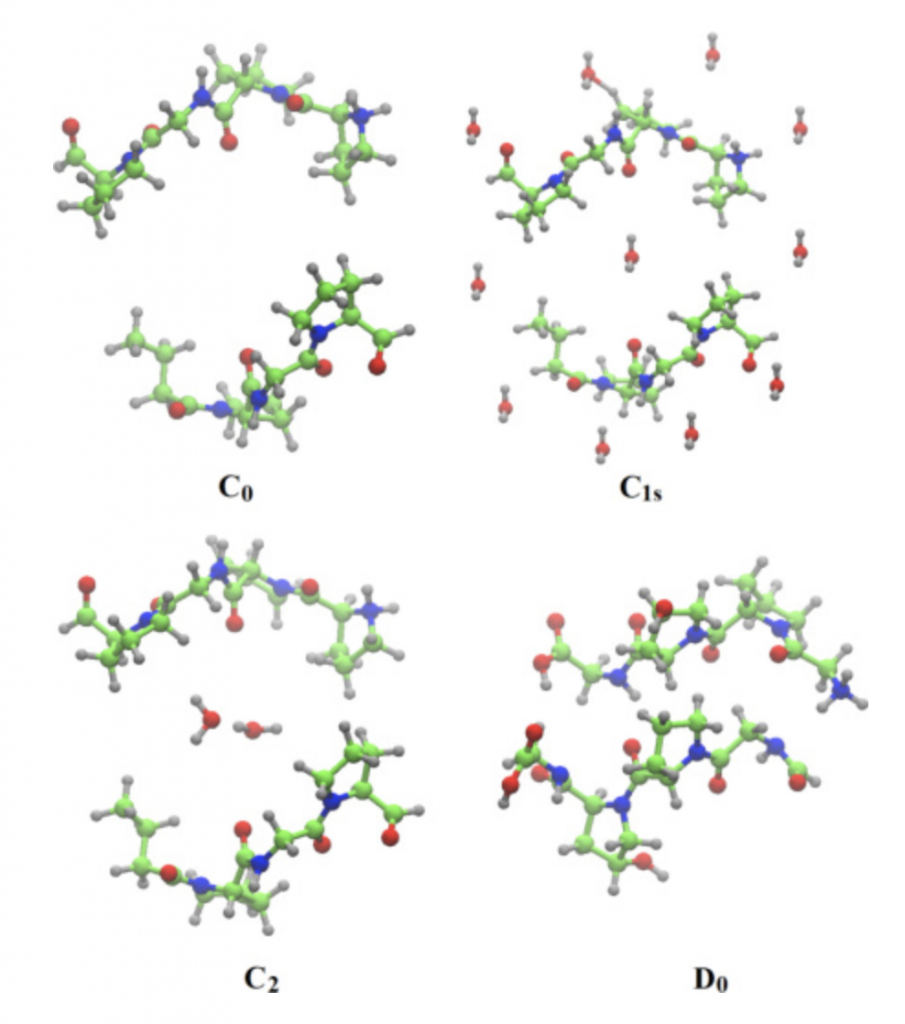 In the present work a detailed vibrational modes assignment of a connective tissue based on the STmod is presented. To the best of our knowledge this is the first report on literature concerning complete vibrational assignment for a tissue. The vibrational calculations were performed on Cn (n – 8), C1s, D0, and D1 unit cells of STmod. The numeric subscript indicates the number of water molecules inside the unit cell. The “s” subscript related to the presence of external water solvating the C1 model. Starting from a hydrated collagen peptide each unit cell was obtained and calculations performed on periodic boundary conditions. The figure on the right shows the unit cell for C0, C1s, C2, and D0 structures.
In the present work a detailed vibrational modes assignment of a connective tissue based on the STmod is presented. To the best of our knowledge this is the first report on literature concerning complete vibrational assignment for a tissue. The vibrational calculations were performed on Cn (n – 8), C1s, D0, and D1 unit cells of STmod. The numeric subscript indicates the number of water molecules inside the unit cell. The “s” subscript related to the presence of external water solvating the C1 model. Starting from a hydrated collagen peptide each unit cell was obtained and calculations performed on periodic boundary conditions. The figure on the right shows the unit cell for C0, C1s, C2, and D0 structures.
See more in https://www.osapublishing.org/boe/abstract.cfm?uri=boe-9-4-1728
New diagnostic tools based on photonic technology have been developed to achieve cancer treatment with favorable results. One of these tools is the optical biopsy. Optical biopsy refers to techniques where the light-tissue interaction is analyzed and the tissue state information is obtained both “in vivo” and “ex vivo”. Biological materials had been successfully characterized using these kinds of techniques. Vibrational spectroscopy, consisting of Raman scattering and Fourier-transform infrared (FTIR) techniques, are of particular interest due of their high sensitivity in the detection of biochemical and molecular changes in biological tissues [7–11].
 In particular, the vibrational modes associated with important biochemical components of cells can be used as markers in the identification of metabolic alterations suffered by the cell during the carcinogenesis process.
In particular, the vibrational modes associated with important biochemical components of cells can be used as markers in the identification of metabolic alterations suffered by the cell during the carcinogenesis process.
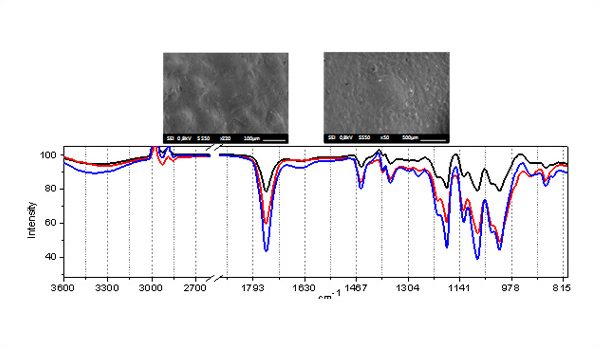
In this paper, alterations in the amide (1500–1700 cm−1 ) spectral region probed by Fourier-transform infrared spectroscopy (FTIR) have been reported comparing tumor and normal tissues. The observed changes in the FTIR spectra of squamous cell carcinoma compared to normal tissues were analyzed by First-Principles Density Functional Theory vibrational calculations. Computational models for skin and prototype β-sheet model were employed. Computational models for the skin model (C0, C1, C4 and D1) and β sheet prototype were used.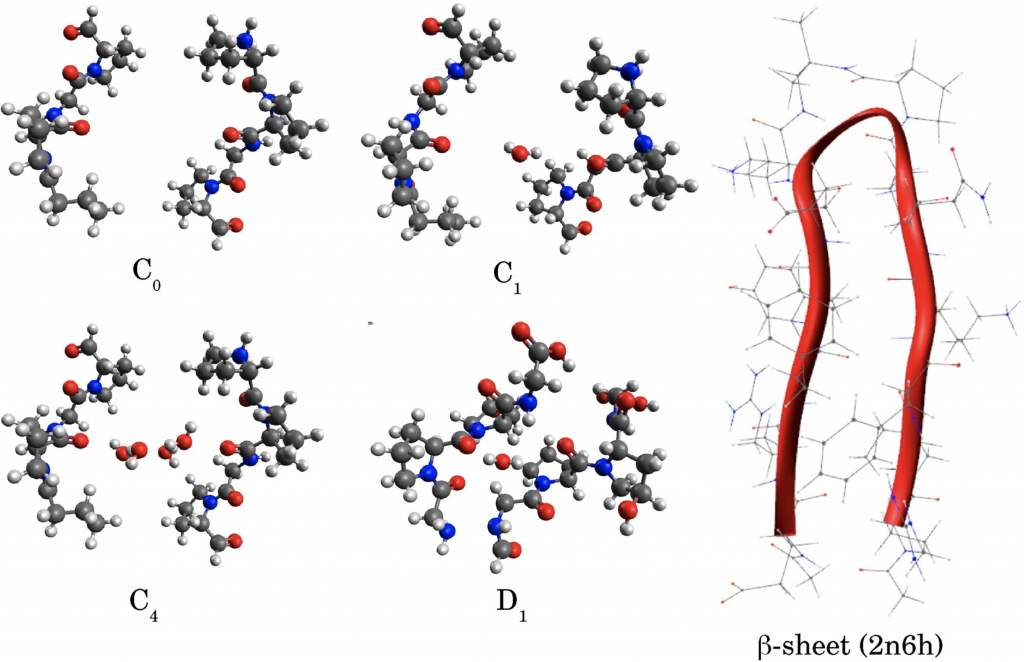
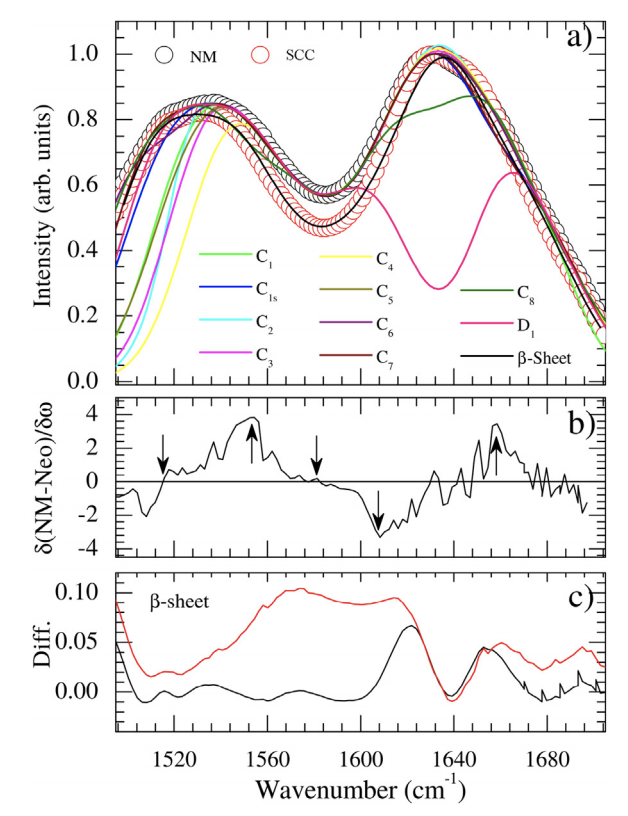 Usually, bands in this range are assigned to the so-called Amide I, II, and III vibrations which provide pieces of information concerning peptide bonds and secondary structure (α-helix, β-sheet) of proteins. Proteins folding changes due to tumoral process are usually considered to qualitatively explain the observed differences between tumor and normal tissues. Our findings shown that predominates conjugated Amide I + Amide II, Amide V, methylene torsions, and ring side chains torsions and swings vibrations in this region. We also notice the lack of evidence concerning changes in the secondary structure of the β-sheet peptidic model to explain the spectral differences. In fact, we concluded that the proline amino acid has the main rule to explain the data in this region being it responsible for the strong coupling between vibrations instead of water.
Usually, bands in this range are assigned to the so-called Amide I, II, and III vibrations which provide pieces of information concerning peptide bonds and secondary structure (α-helix, β-sheet) of proteins. Proteins folding changes due to tumoral process are usually considered to qualitatively explain the observed differences between tumor and normal tissues. Our findings shown that predominates conjugated Amide I + Amide II, Amide V, methylene torsions, and ring side chains torsions and swings vibrations in this region. We also notice the lack of evidence concerning changes in the secondary structure of the β-sheet peptidic model to explain the spectral differences. In fact, we concluded that the proline amino acid has the main rule to explain the data in this region being it responsible for the strong coupling between vibrations instead of water.
See more in https://www.sciencedirect.com/science/article/abs/pii/S0924203118301371
There is a growing interest in exploiting the ability to synthesize gold nanoparticles (GNPs) of controlled size and shape mediated by amino acids and peptides. For many years, numerous synthetic strategies developed for the synthesis of GNPs have frequently required the use of harsh reagents for controlling the particle morphology. The search for eco-friendly conditions showed that peptides are an interesting alternative for reducing the environmental impacts during the synthesis of GNPs. Also, the capping of GNPs provided by peptides assures efficient delivery and biocompatibility that can contribute to the use of theranostic properties of these materials.8 Moreover, the functional groups available in the peptides, such as −SH and −NH2, allow a fine control of the synthesis for achieving the desired functionality for the material. Peptides provide templates that direct the growth and shape of the metal nanoparticles as well as their capping. Particularly, the presence of a cysteine residue in a peptide provides a mild reducing agent for biomineralization and an efficient anchor for the peptide on the GNP surface. Therefore, a peptide containing a cysteine residue is a promising compound for the controlled synthesis and capping of GNPs. The manipulation of the reaction conditions and a rational choice of the amino acids arrangement (bioinspired sequences) make feasible the specific directing of the GNP spatial growth assisted by peptides. Bioinspired peptides have been shown to be useful for the assembly and synthesis of gold nanostructures.
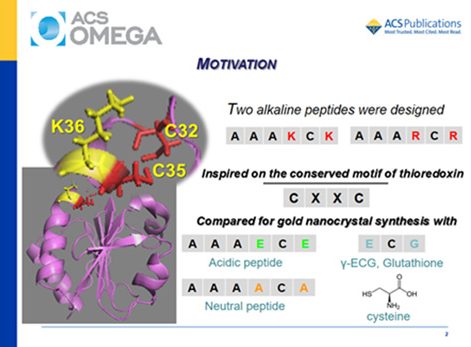
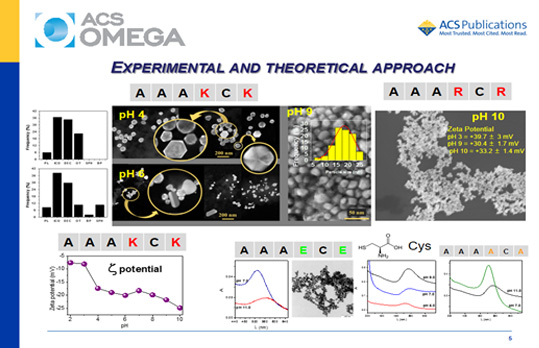
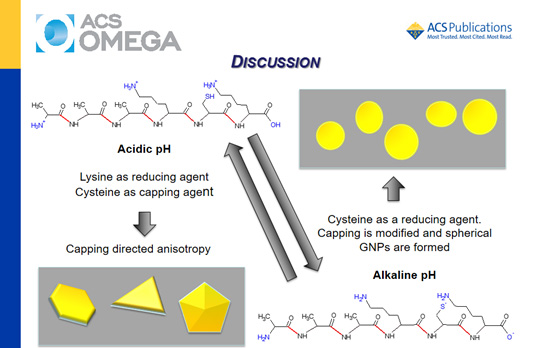
Alkaline peptides AAAXCX (X = lysine or arginine residues) were designed based on the conserved motif of the enzyme thioredoxin and used for the synthesis of gold nanoparticles (GNPs) in the pH range of 2–11. These peptides were compared with free cysteine, the counterpart acidic peptides AAAECE and γ-ECG (glutathione), and the neutral peptide AAAACA. The objective was to investigate the effect of the amino acids neighboring a cysteine residue on the pH-dependent synthesis of gold nanocrystals. Kohn–Sham density functional theory (KS-DFT) calculations indicated an increase in the reducing capacity of AAAKCK favored by the successive deprotonation of their ionizable groups at increasing pH values. Experimentally, it was observed that gold speciation and the peptide structure also have a strong influence on the synthesis and stabilization of GNPs. AAAKCK produced GNPs at room temperature, in the whole investigated pH range. By contrast, alkaline pH was the best condition for the synthesis of GNP assisted by the AAARCR peptide. The acidic peptides produced GNPs only in the presence of polyethylene glycol, and the synthesis using AAAECE and γ-ECG also required heating. The ionization state of AAAKCK had a strong influence on the preferential growth of the GNPs. Therefore, pH had a remarkable effect on the synthesis, kinetics, size, shape, and polydispersity of GNPs produced using AAAKCK. The AAAKCK peptide produced anisotropic decahedral and platelike nanocrystals at acidic pH values and spherical GNPs at alkaline pH values. Both alkaline peptides were also efficient capping agents for GNPs, but they produced a significant difference in the zeta potential, probably because of different orientations on the gold surface.
See more in pH-Dependent Synthesis of Anisotropic Gold Nanostructures by Bioinspired Cysteine-Containing Peptides.
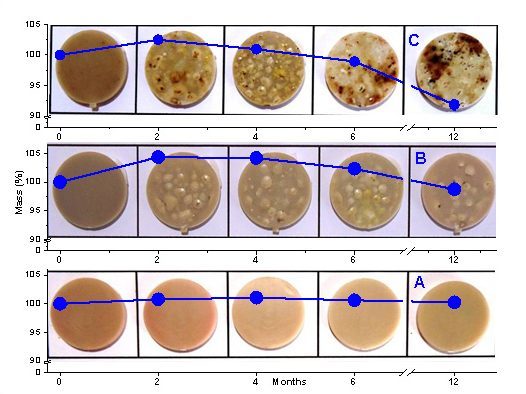
In an other study, poly(lactic acid) (PLA) and their blends with 5%/wt and 10%/wt thermoplastic starch (TPS) were submitted to degradation in simulated soil. To investigate the mechanisms involved in the degradation, we also submitted the samples to degradation by tert-butyl hydroperoxide, myoglobin, and peroxide-activated myoglobin. The samples were analyzed by Fourier-transformed infrared spectrometry (FTIR), scanning electronic microscopy (SEM), contact angle analysis, and mass loss measurement. The FTIR results indicated a weak interaction between the two components (PLA and starch) in the blend’s amorphous structure. However, the corresponding SEM images showed that TPA increased ridges and roughness at the material surface associated with an increase of wettability evidenced by contact angle analysis. Consistently, TPS favored degradation of the material both in the simulated soil and pro-oxidant model systems. In the simulated soil, the occurrence of TPS hydrolysis provided glucose, a biological fuel, that contributed to the growth of the microorganisms. The similar degradation patterns observed in mimetic pro-oxidant biological systems and soil suggest that oxidative reactions catalyzed by heme proteins from biological sources as well as the presence of peroxides and transition metal traces in the original materials have a significant contribution to PLA and PLA/TPS degradation. See the article in Biological Oxidative Mechanisms for Degradation of Poly(lactic acid) Blended with Thermoplastic Starch publicado em ACS Sustainable Chem. Eng. 2015.